Primer Nature Review of Alpha 1 Antitrypsin Deficiency
- Review
- Open Access
- Published:
Alpha-1 antitrypsin (AAT) augmentation therapy in individuals with the PI*MZ genotype: a pro/con debate on a working hypothesis
BMC Pulmonary Medicine book 21, Commodity number:99 (2021) Cite this article
Abstruse
Blastoff-1 antitrypsin deficiency (AATD) is a significantly under-diagnosed genetic condition caused by reduced levels and/or functionality of alpha-one antitrypsin (AAT), predisposing individuals to lung, liver or other systemic diseases. The direction of individuals with the PI*MZ genotype, characterized by mild or moderate AAT deficiency, is less clear than of those with the most mutual severe deficiency genotype (PI*ZZ). Contempo genetic data propose that the PI*MZ genotype may be significantly more than prevalent than currently idea. The only specific handling for lung affliction associated with astringent AATD is the intravenous infusion of AAT augmentation therapy, which has been shown to tedious disease progression in PI*ZZ individuals. At that place is no specific prove for the clinical benefit of AAT therapy in PI*MZ individuals, and the risk of emphysema development in this group remains controversial. As such, electric current guidelines exercise not support the apply of AAT augmentation in PI*MZ individuals. Here, we hash out the limited information on the PI*MZ genotype and offer pro and con perspectives on pursuing an AAT-specific therapeutic strategy in PI*MZ individuals with lung affliction. Ultimately, further research to demonstrate the rubber, risk/benefit balance and efficacy of AAT therapy in PI*MZ individuals is needed.
Background
Most of the published prove relating to the management of individuals with blastoff-1 antitrypsin deficiency (AATD) is based on patients with the PI*ZZ or PI*Znull genotypes, who have a severe deficiency in blastoff-1 antitrypsin (AAT), with plasma levels < 11 µM (< 52 mg/dL) compared with the normal range of 19–47 µM (102–254 mg/dL) [1]. Nevertheless, there are an estimated vi–7 million people with AATD in the United States (US) alone, including those with mild or moderate deficiency genotypes [2]. Individuals who are heterozygous for the Z allele, such as those with the PI*MZ genotype, who have AAT serum levels of 11–28 µM (62–151 mg/dL), or approximately threescore% of the normal range [1], may exist at risk of developing lung and/or liver disease if they have other predisposing risk factors. Previous studies have found that amongst patients diagnosed with chronic obstructive pulmonary affliction (COPD), the prevalence of the PI*MZ genotype ranges from 1 to 22% [iii, four].
Despite the understanding of the mechanisms responsible for pathologic changes in AAT-scarce individuals [5], and the fact that AAT augmentation therapy is the simply illness-modifying therapeutic approach for patients with AATD-associated lung illness [vi], current guidelines do non recommend the employ of augmentation therapy in individuals with the PI*MZ genotype. However, there is a lack of consensus amidst treating physicians on how PI*MZ individuals should be monitored and treated, and whether AAT augmentation therapy could exist a handling strategy in some PI*MZ patients. Hither, nosotros summarize the evidence supporting a potential AAT-specific therapeutic approach in individuals with the PI*MZ genotype, and also discuss reasons why focus on this arroyo may not be warranted.
Main text
Pro: AAT treatment of PI*MZ patients could be an option
Focus on AAT serum concentrations in PI*MZ tin be misleading
1) The relevance of the 'protective threshold'
Systemic levels of AAT in PI*MZ individuals practice non generally autumn below 11 µM, a value historically used as a theoretical 'protective threshold' for AAT therapy provision; levels beneath this threshold are thought to be associated with a higher risk of developing emphysema [7]. In that location are, however, several issues with this theoretical threshold as a reason to care for or withhold AAT augmentation therapy. The 'protective threshold' of 11 µM was chosen based on historical data fatigued from standards that lacked acceptable accuracy and clinical validation [seven, 8]. The threshold represents a systemic concentration of AAT in serum rather than its level in pulmonary epithelial lining fluid, and thus, does not necessarily accurately reflect AAT functional activity in the lung microenvironment. Furthermore, this threshold concentration is based on nephelometric measurement of antigenic, rather than functional, action, which may exist lower due to misfolded, dysfunctional proteins in some individuals [nine, 10]. Normalizing AAT serum levels in patients with severe AATD with doubling the standard dose of augmentation therapy resulted in a significant reduction in inflammatory markers compared with traditional dosing [eleven], further questioning the approach to using a threshold value to dichotomize the problem and decide the direction. At the aforementioned time, the PI*MZ phenotype is associated with a wide range of abnormal AAT levels. While the 2003 European Respiratory Society/American Thoracic Order argument indicates that AAT levels are only mildly reduced in those with the PI*MZ phenotype (17–33 µM, or 90–210 mg/dL) [iii], two screening studies reported much lower levels, with similar ranges of ~ 11.9 to 29.0 µM (62–151 mg/dL) [12] and ~ 12.seven to 19.2 µM (66–100 mg/dL) [13]. In add-on, it is known that heterogeneity of disease exists contained of AAT serum levels. For example, PI*ZZ individuals who have reduced serum levels may be asymptomatic or have balmy symptoms of lung disease, while others may take severe lung disease [14], suggesting that the relationship between AAT serum levels and presentation of affliction may not e'er be anticipated.
Although there are substantial limitations to the 'protective threshold', information technology should be noted that a solid alternative to this does not be today. A shift toward a model of AAT deficiency based on functionality—rather than quantity—is warranted; however, quantitative AAT serum assays are widely used and inexpensive, while assays assessing AAT functional activity [xv] are utilized in only a handful of specialized laboratories globally. Therefore, progress towards an inexpensive, reproducible, and widely available AAT activity assay is required.
2) Steady-state versus pro-inflammatory astute settings
The traditional evaluation of steady-state serum levels may atomic number 82 to misinterpretation of its findings in two unlike means. Inducible plasma levels of AAT increment in comparison to steady-land levels [16,17,eighteen], and equally an astute phase reactant, AAT contributes in limiting local and systemic inflammation [19]. The anti-inflammatory and cytoprotective effects of AAT are of high importance in acute inflammatory conditions, where increased levels of neutrophil elastase (NE) atomic number 82 to compromised lung permeability and induce the release of pro-inflammatory cytokines [19, xx]. Thus, adequate allowed response in acute inflammation may be, to a certain extent, dependent on advisable AAT increment, which is compromised in patients with AATD, including heterozygous PI*MZ individuals. On the other manus, unrecognized inflammation at the time of presumed "steady-land" measurement of AAT levels tin can mask the bodily level of AAT deficiency and pb to overestimation of baseline AAT levels, compromising the power to identify PI*MZ individuals at run a risk of progressive lung function deterioration [21]. Data from a national AAT deficiency-targeted screening accomplice showed that approximately a quarter of PI*MZ samples showed signs of inflammation, as evidenced past increased levels of C-reactive poly peptide (CRP) ≥ five mg/Fifty [21]. This indicates that the actual 'steady-state' levels of AAT in these individuals when inflammation is not nowadays may exist lower than measured. In add-on, AAT and CRP serum levels are elevated in not-deficient COPD subjects, suggesting an increased level of systemic inflammation in COPD and that increased levels of AAT may be a physiologic response to compensate for this increased inflammation [22].
3) AAT levels versus protease-antiprotease remainder
While we acknowledge the relevance of measuring and interpreting serum AAT levels, the ultimate goal of AAT augmentation therapy is to restore the balance of proteases and antiproteases in these patients in the long term. PI*MZ individuals mostly take a more than favorable protease-antiprotease rest than PI*ZZ individuals due to college levels of AAT. Withal, the deficiency of functional AAT in PI*MZ individuals, and the accumulation of misfolded/non-functional AAT, may increase inflammation in the lungs due to the reduced inhibition of NE and increased chemoattractant production, that could facilitate neutrophil activation and increment enzyme activity, exacerbating lung affliction in some individuals (Fig. 1) [23]. It is also nether-appreciated that AAT inhibits proteases other than NE, such as proteinase-3, and has important immunomodulatory functions [11, 24, 25], interruption of which may also be disease-causing. Infusions of AAT in severely deficient individuals reduces leukotriene B4 (LTB4) and NE activeness in the lung, besides every bit reducing a range of pro-inflammatory cytokines [11, 26]. Sputum analysis of PI*MZ subjects without airflow obstruction identified inteleukin-viii (IL-viii)-related neutrophilic inflammation in the airways, similar to stable COPD patients, suggesting an increased adventure of progressive pulmonary changes related to the pro-inflammatory consequences of raised neutrophil levels in individuals with reduced functional AAT [27]. Furthermore, the germination of Z polymers, particularly at the sites of inflammation, could dilate the allowed response and may crusade further damage leading to emphysema [28].
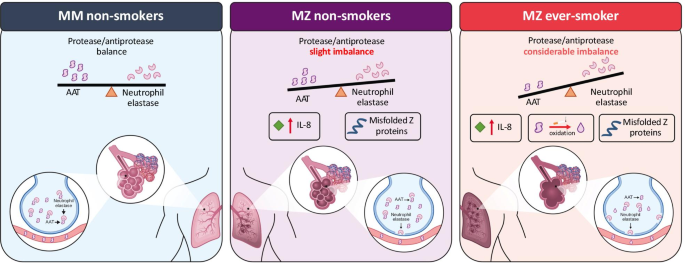
Potential mechanism for increased disease risk in individuals with the PI*MZ genotype. Left panel: In MM non-smokers, a normal protease/antiprotease remainder exists with normal alveoli. Key console: MZ non-smokers have a slight imbalance of AAT and neutrophil elastase, IL-8 levels may increment in the lungs causing neutrophil activation and inflammation and progressive damage in the lungs in some patients. Correct panel: MZ smokers have a greater imbalance between AAT and neutrophil elastase as a outcome of reactive oxygen species in cigarette smoke inactivating AAT. Additional factors such as Z polymers and IL-viii inflammatory markers cause greater production of neutrophil elastase in the lung, causing irreversible damage. Effigy adapted from Carroll et al. 2014; https://doi.org/10.5772/58602 [23] under the Creative Commons Attribution iii.0 License. AAT blastoff-1 antitrypsin, IL interleukin
The imbalance between AAT and proteases may not only be related to AAT levels; another factor impacting affliction hazard in PI*MZ individuals is the genetics underpinning the expression of NE. A recent study in AATD and control subjects explored the expression of the neutrophilic elastase-encoding cistron (Elastase, Neutrophil Expressed [ELANE]), which provides another indication of the degree of proteinase and antiprotease balance [29]. Expression of ELANE was found to exist greatly variable, with the highest levels shown in PI*MM controls. In subjects with the PI*ZZ genotype, ELANE expression was shown to correlate with lung function, suggesting that in individuals with low AAT levels, ELANE expression is an boosted modifier of protease/antiprotease imbalance and illness risk [29]. Therefore, theoretically, PI*MZ individuals with loftier levels of ELANE expression and reduced AAT levels may have significant protease/antiprotease imbalance and increased risk for developing COPD in comparison to PI*MZ individuals with normal ELANE or even PI*ZZ individuals with lower levels of ELANE. Focus on correcting the remainder between proteases and antiproteases rather than the focus on actual levels of AAT may explain possible beneficial effects of AAT augmentation therapy in not-scarce individuals with cystic fibrosis and pneumonia, where AAT inhalation led to elastase inhibition and a reduction in the downstream effects of inflammation [25]. Following a similar concept, ongoing studies are evaluating the benefits of the inhibition of NE activity in AAT-deficient patients who are non treated with augmentation therapy [24, 25, 30].
PI*MZ individuals develop illness as a consequence of AATD
Several example–control studies indicated a higher prevalence of the PI*MZ genotype amid patients with COPD compared with controls without COPD [31,32,33]. A recently published analysis of a large cohort of individuals with COPD showed that PI*Z heterozygotes with a pregnant cigarette smoking history are at an increased risk of COPD compared with e'er-smoker PI*MM individuals, take lower lung function, greater airflow obstruction and greater computed tomography (CT)-based quantitative measures of emphysema [34, 35]. These reports suggest that PI*MZ patients have a higher susceptibility to smoking-related lung disease. Even so, like to the fact that not all PI*ZZ individuals suffer from clinically pregnant lung or liver disease during their lifetime, information technology is evident that many PI*MZ individuals exercise non suffer from extensive lung or liver disease.
Rationale for augmentation therapy in non-severely AAT-deficient individuals
Preventing lung function refuse is one of the primal goals in the management of COPD, and information show that forced expiratory volume in i s (FEV1) reject and exacerbation rates are significantly associated with outcomes in this disease [36]. Current guidelines recommend AAT augmentation therapy in patients with severe AATD (ZZ or Zip genotypes) with airflow obstruction [37,38,39], with additional criteria to select the handling candidates, including smoking abeyance, FEV1 ≤ 65% and AAT serum levels ≤ eleven µM [38, 40]. In common practice, FEV1 decline [38] or exacerbation frequency [41] may influence the decision to consider initiation of the augmentation therapy in patients with severe AAT deficiency, even before all other criteria for the initiation of AAT are fulfilled [42].
In patients with astringent AATD, "rapid decliners" can accept an FEV1 loss of over 200 mL/year [43, 44]. Analysis of ii independent studies found a 3.9% lower FEV1/forced vital capacity (FVC) ratio in PI*MZ compared with PI*MM individuals, after adjusting for pack-years, age, sex, and height, suggesting that some patients with the PI*MZ genotype may have a slight increased risk of developing AATD-related COPD compared with PI*MM individuals [45]. Understanding tremendous heterogeneity in the rate of FEV1 decline and survival, despite assistants of recommended management approaches in COPD [36], it is clear that a prevalent population of PI*MZ individuals with COPD [3, 4, 35] includes individuals with progressive affliction despite other management strategies being adequately practical. Observational show suggests that AAT augmentation therapy may be specially effective in patients with severe AATD [44], and this may provide a basis to consider treating patients with heterozygous genotypes (such as PI*MZ patients) and rapid lung part decline with AAT. When investigating heterogeneity in lung function refuse in PI*MZ individuals, it is important to admit lack of sensitivity of routine spirometry to capture emphysema progression in patients with AATD [7], and additional tests such as diffusing capacity and quantitative imaging may exist required in future studies.
From a practical standpoint, recent data propose that a significant number of individuals in the U.s. diagnosed with the PI*MZ genotype are prescribed AAT therapy, contrary to electric current indications. In an analysis of the AlphaNet disease direction and prevention program (ADMAPP), out of a total of 3506 individuals, the majority of whom were receiving AAT therapy (bodily numbers not specified), ~ 13% of patients were reported to accept the PI*MZ genotype [46]. While nosotros may speculate that a significant number of these individuals may have been started on this therapy simply due a lack of adherence to the guidelines, and recognize that handling is against recommendations, the number of PI*MZ patients receiving AAT therapy in the US may provide a platform for retrospective studies on the clinical efficacy of augmentation therapy in this patient population.
Ethical considerations
There is an overall consensus that current recommendations to merely treat severe AATD are, to certain extent, based on the lack of evidence for the clinical benefit of augmentation therapy in a larger population of AATD patients across the PI*ZZ genotype. Yet, given that in that location is good evidence for efficacy in an adjacent group, ethical questions arise in terms of not exploring treatment of PI*MZ patients who take a clinical state that mirrors that of PI*ZZ individuals. Furthermore, it is known that not all PI*ZZ individuals receiving AAT augmentation benefit from therapy, and in the same manner, although not all PI*MZ may benefit from augmentation therapy, a proportion of patients may accept improved clinical outcomes.
In add-on, the consensus to but treat PI*ZZ individuals has resulted in phenotypes other than PI*ZZ or PI*Null being less adequately screened for, and may have contributed to a situation where specific interventions for less severe forms of AATD have not been well explored. This may have been exacerbated past not investigating 'proof-of-concept' for efficacy of AAT therapy in PI*MZ individuals, possibly slowing progression towards a more cost-effective handling with a like mechanism of activeness.
Conclusions
AAT is a powerful protein with multiple immunomodulatory functions. Its deficiency—severe, as seen in PI*ZZ or PI*Null individuals, or moderate, as nigh oft seen in PI*MZ individuals—represents an abnormal country that leads to a compromised response to inflammation and predisposition to a affliction state. A portion of PI*MZ individuals who, despite lifestyle modifications and elimination of take a chance factors with adequate non-specific treatment regimens, continue to have significant lung function deterioration, could benefit from the handling of this genetic disorder, and AAT augmentation therapy might be an option in their management. Further research on this topic is warranted.
Con: PI*MZ individuals should non exist treated with AAT augmentation therapy
Disease risk and severity in patients with the PI*MZ genotype
It is well known that smoking is a key take chances factor for the development of lung affliction in patients with AATD, and disease progression and survival are both significantly worse in smokers than never-smokers [iii]. This also applies to those with the PI*MZ genotype, with a family unit-based study showing that cigarette smoke exposure influenced the take chances for impaired lung part and COPD, while PI*MZ individuals who had never smoked did not develop lung illness [35]. Furthermore, this study plant that PI*MZ smokers accept a higher chance of COPD in comparison to PI*MM smokers [35]. Interestingly, this difference may be express to those with a low smoking history. Another study institute that PI*MZ individuals with a smoking history of < 20 pack years had more severe emphysema on CT scan than equivalent PI*MM individuals, simply this divergence was not credible between PI*MZ and PI*MM individuals with higher levels of smoking (> 20 pack years) [45]. Information technology is therefore thought that PI*MZ individuals accept a small increased gamble for COPD compared with PI*MM individuals, with a minor proportion of individuals having a greater hazard of developing COPD, likely equally a outcome of additional environmental or genetic adventure factors [47]. Contempo data from patients with non-AATD COPD showed that all levels of smoking exposure are associated with lasting and progressive lung damage, with the pass up in lung office only normalizing 20 years afterward smoking abeyance in some patients (Fig. 2) [48]. Given the increased affect of smoking in patients with AATD, these data emphasize the importance of encouraging smoking cessation to prevent deterioration in lung office.
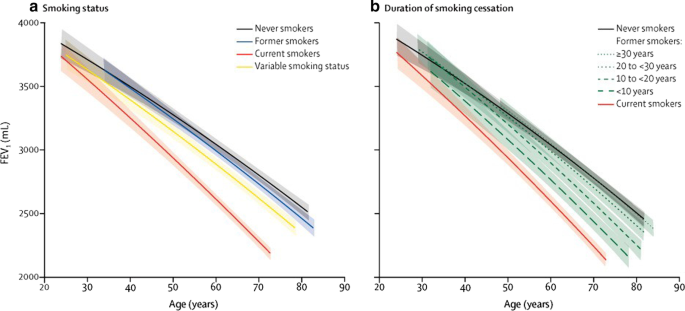
Predicted FEV1 curves according to smoking status (a) and duration of smoking cessation (b) [48]. Effigy reproduced with permission from Oelsner et al. Lancet Respir Med 2020;8:34–44, Copyright Elsevier 2020. FEV 1 forced expiratory volume in ane south
While the clinical course of disease for PI*MZ heterozygotes is less clear than the PI*ZZ genotype, the majority of individuals with the PI*MZ genotype, especially in the absence of boosted risk factors, will not develop significant lung affliction that ofttimes characterizes PI*ZZ or PI*Null genotypes. A meta-analysis, which included 7 cross-sectional studies reporting lung function as a continuous outcome, found no difference in hateful FEVone% predicted between PI*MZ and PI*MM individuals (p = 0.62) [47]. Furthermore, information from a longitudinal community study suggested that having the PI*MZ genotype was non a significant adventure factor for an accelerated decline in FEV1 [49]. It has too been reported that patients with COPD and the PI*MZ genotype exercise not have an increased adventure of COPD hospitalization, unless the patient had a first-degree relative with emphysema and the PI*ZZ genotype [50]. This study suggests that, in addition to the Z allele, other genetic or environmental factors that contribute to lung disease development remain unknown.
Exposure to occupational and environmental pollutants that cause respiratory irritation (eastward.g., gases/fumes used in the agricultural industry) are known to be independent hazard factors for lung role impairment in PI*MZ individuals, and should exist avoided where possible to assistance maintain expert lung wellness [51, 52]. There is, still, accumulating evidence for an increased susceptibility to occupational exposure-related lung function refuse in PI*MZ individuals who are current or ex-smokers [37, 51]. In summary, individuals with the PI*MZ genotype who are non-smokers practise not take an increased take chances for COPD, and only a subset of individuals may be more susceptible due to other genetic or environmental factors [53]. Fugitive risk factors such equally smoking is crucial in these individuals; notwithstanding, the full touch of other genetic or environmental factors needs farther clarification in larger studies.
While the currently available information on disease adventure in PI*MZ individuals are sourced from well-designed studies and are mostly consistent, it should exist noted that a limitation is that they are largely based on FEVone. Although FEV1 is a reproducible and cost-effective endpoint, it lacks sensitivity to quantify emphysema progression. Farther data, based on diffusing capacity, CT or other clinical outcomes, such as exacerbations, would help clarify affliction risk in PI*MZ individuals.
AAT therapy: current state of the evidence
It has previously been noted that the biological rationale for the provision of AAT therapy in PI*MZ individuals is unclear; with plasma AAT levels effectually lx% of normal, individuals have much higher AAT levels than patients with severe disease (approximately twenty% of normal) [3, five]. Patients are therefore considered to take plasma levels considerably college than what is currently deemed the 'protective threshold' (11 µM), and provision of AAT augmentation therapy for these patients would therefore appear to be unnecessary based on levels of AAT solitary. A longitudinal clinical trial investigating the efficacy of AAT therapy in patients with PI*MZ AATD and significant respiratory dysfunction would be required to determine efficacy in this population. However, it is debatable whether this is warranted based on what is known regarding the disease risk in this population and the strong influence of smoking on disease progression. At this time, in that location are no data supporting the use of AAT therapy in patients with the PI*MZ genotype (which is reflected in current guidelines/position statements [37, 38, xl]) and limited rationale to pursue this as a therapeutic approach in the future. In one case we have a wider agreement of the specific phenotypic characteristics related to non-astringent PI*MZ, and whether AAT deficiency is a cause or contributing cistron to evolution of chronic lung disease, the appropriateness of the employ of available specific therapies can be evaluated.
Ethical considerations
Information technology is important to consider that the PI*MZ genotype is much more common than the PI*ZZ genotype, with a worldwide prevalence of half-dozen.2% versus 0.02%, respectively [54]; therefore, it could be argued that the high prevalence of the PI*MZ genotype could potentially create shortages in the provision of AAT therapy in these patients. It is important to annotation that AAT is a plasma-derived protein, is in limited supply, and is costly [55]. Therefore, providing unnecessary handling to large numbers of PI*MZ individuals poses the risk that treatment becomes less accessible for patients with more than astringent deficiency, who have shown a clear do good from treatment. Although it could exist argued that supply of AAT should exist directed towards patients who bear witness a response to treatment rather than based on genotype, assessing response to treatment should be ideally assessed over several years, requiring comprehensive, well-structured prospective studies. Furthermore, arguments for investigating handling in PI*MZ individuals should exist viewed in the current context, where a significant number of patients with a severe genotype eligible for treatment probable remain unidentified. Efforts should be focused on identifying and treating individuals who are almost likely to benefit from augmentation therapy with the potential to change the grade of disease, rather than heterozygous individuals in whom the clinical outcomes of AAT therapy are non nonetheless clear. Moreover, focusing on the limitations of current measures, such as the precise relevance of the 'protective threshold', could upshot in a paradigm shift in terms of acceptance that current understanding of AATD is wrong, which could also cast doubt on the decision to treat homozygous individuals. Many countries/healthcare systems do not deem AAT therapy to be cost-effective and practise not reimburse treatment of patients with severe deficiency, despite current recommendations [37, 42]. Furthermore, in patients with genotypes for astringent disease, at that place are data on which to base cost-effectiveness predictions; however, there are no such data bachelor for patients with the PI*MZ genotype. Therefore, pursuing an AAT-specific treatment strategy in patients who may non demand the treatment may put an undue financial burden on healthcare systems and individuals. Information technology is as well important to consider that treatment with AAT therapy is normally lifelong. This may advise that some patients with the PI*MZ genotype could receive unnecessary, cumbersome intravenous treatment, which may become a brunt and reduce patient quality of life.
Conclusions
Evidence suggests that COPD in individuals with the PI*MZ genotype is mainly driven by smoking, and these individuals are only at a slightly higher take chances of lung disease than equivalent smoking PI*MM individuals. There is, therefore, little rationale for treatment of PI*MZ patients to differ from that of similar PI*MM patients. Electric current guidelines exercise not recommend the treatment of PI*MZ individuals with AAT therapy due to the absence of specific evidence for utilize in this population showing that potential benefits outweigh safety risks. Sufficiently powered randomized controlled trials would exist required to provide this show; withal, there is little rationale to justify the establishment of such a study. As such, preventative measures, principally highlighting the risks of smoking and encouraging patients to stop or avert starting smoking, may exist the most constructive treatment for individuals with the PI*MZ genotype. It is of import to annotation that smoking cessation in COPD in general is a disease-modifying intervention [56]; lung function decline is known to stabilize after smoking abeyance in the bulk of COPD patients [57, 58].
Word
The fundamental factor that currently precludes the treatment of PI*MZ individuals with AAT therapy is the lack of evidence supporting the benefits of the augmentation. This lack of bear witness is at to the lowest degree partially related to the potential for creating a substantial burden in terms of healthcare resources and medication supply, as well as the handling-related burden of life-long intravenous infusions. While early identification of AATD is important to encourage lifestyle change, it remains to exist adamant whether lower levels of AAT, compared with normal levels seen in PI*MM individuals, are associated with a disease risk and emphysema/COPD progression, justifying the need for therapy. Electric current guidelines do not recommend use of AAT augmentation therapy in PI*MZ patients and merely provide recommendations for therapy in patients with severe AATD (ZZ or Null genotypes) with emphysema [37,38,39]. Nevertheless, information technology remains a possibility that some individuals with the PI*MZ genotype and accelerated lung part reject may benefit from augmentation therapy; further investigation would be needed to determine the effects of handling in these individuals. However, it is important to note that, at nowadays, the lack of excessive lung office turn down in patients should not in itself forestall the use of AAT therapy in severe illness (eastward.yard., PI*ZZ individuals), and a holistic approach should be utilized; importantly, emphysema progression and morphology/distribution should be considered. This would seem to lessen the argument regarding the relevance of rapid lung office decline in PI*MZ individuals; however, it is unlikely that any patient experiences rapid decline consistently through life, with the detection of rapid decline possibly providing a 'snapshot' indicating an underlying issue that may warrant treatment. Evolution of future therapies, which could reduce the costs, lack of availability and cumbersomeness of augmentation therapy, will allow us to re-evaluate the paradigm regarding severe vs. not-severe AATD, and potentially expand our handling goals in AATD.
Furthermore, it is important to note that while the Z allele is nigh commonly associated with AATD, this variant is only one of numerous variants that have been linked to AATD. Many rare/novel/goose egg alleles are difficult to detect or are non-detectable by almost testing methods (e.g., isoelectric focusing [IEF] and targeted polymerase chain reaction), and can just exist detected by genetic sequencing, the availability of which is highly variable [59]. In particular, many rare alleles are Yard-like (with like IEF banding blueprint to the wild-blazon Grand protein). As such, many PI*MZ individuals with lower AAT levels and COPD may, in fact, be compound heterozygotes for the Z allele and a rare/M-similar allele [59]. A recent study has reported a high rate of rare deficiency alleles in individuals who were previously identified as PI*MZ [threescore], which raises the question of what proportion of PI*MZ patients with severe disease/fast decline are 'true' PI*MZs. The challenges to accurately diagnosing rare/M-similar Z compound heterozygotes include a lack of awareness and ability to detect rare alleles (east.g., with gene sequencing).
Although not the focus of the present newspaper and not relevant to the provision of AAT therapy, there is also increasing prove that the PI*MZ genotype is a take a chance factor for liver disease in terms of clinically significant portal hypertension and low-course liver fibrosis [61]. Published literature consistently identifies heterozygous PI*Z individuals to have increased take a chance for cirrhosis and liver failure requiring transplantation [62, 63]. Smaller retrospective studies propose that a primary liver carcinoma might develop even in a heterozygote country of PI*Z AATD, even without concurrent liver disease [64]. Thus, it is important to recognize that the relevant problem in the PI*MZ population is not to prove their evidently lower take a chance of disease development in comparing to PI*ZZ individuals, but rather to understand their most likely increased run a risk of affliction evolution in comparison to the population of AAT non-deficient individuals with the same risk factors. This boosted risk supports the importance of identifying PI*MZ individuals in guild to advise on preventative measures such as hepatitis vaccination, in improver to smoking abeyance.
Conclusions
Several points for and against the provision of AAT augmentation therapy in patients with the PI*MZ genotype have been presented (Table i). Arguments discussed here do not suggest that augmentation therapy should exist administered to PI*MZ patients, only it is articulate that in that location is a gap in knowledge regarding the utility of AAT therapy in PI*MZ individuals. Still, as the PI*MZ genotype is a relatively mutual risk factor for COPD, it tin exist agreed that the recognition and identification of the PI*MZ genotype is of ultimate importance, as lifestyle modifications can substantially influence the clinical course of illness. The provision of AAT-specific therapy to patients with this genotype would exist dependent on farther prospective studies focused on amend understanding the natural history of PI*MZ AATD, and studies evaluating the response of PI*MZ individuals to augmentation therapy.
Availability of data and materials
Not applicative.
Abbreviations
- AAT:
-
Alpha-1 antitrypsin
- AATD:
-
Blastoff-i antitrypsin deficiency
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- ELANE:
-
Elastase, neutrophil expressed
- FEVone :
-
Forced expiratory volume in ane s
- FVC:
-
Forced vital capacity
- NE:
-
Neutrophil elastase
References
-
Hurley K, O'Connor GT. Serum alpha1-antitrypsin concentration in the diagnosis of alpha1-antitrypsin deficiency. JAMA. 2018;319(19):2034–5.
-
de Serres FJ, Blanco I. Prevalence of alpha1-antitrypsin deficiency alleles PI*Southward and PI*Z worldwide and effective screening for each of the 5 phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis. 2012;6(five):277–95.
-
American Thoracic S, European RS. American Thoracic Guild/European Respiratory Society argument: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(vii):818–900.
-
Al Ashry HS, Foreign C. COPD in individuals with the PiMZ blastoff-1 antitrypsin genotype. Eur Respir Rev. 2017;26(146):170068.
-
de Serres F, Blanco I. Role of alpha-1 antitrypsin in human health and affliction. J Intern Med. 2014;276(iv):311–35.
-
McElvaney NG, Burdon J, Holmes M, Glanville A, Wark PA, Thompson PJ, et al. Long-term efficacy and safety of alpha1 proteinase inhibitor handling for emphysema acquired by severe alpha1 antitrypsin deficiency: an open up-label extension trial (RAPID-OLE). Lancet Respir Med. 2017;5(1):51–sixty.
-
Brantly ML, Lascano JE, Shahmohammadi A. Intravenous alpha-1 antitrypsin therapy for alpha-ane antitrypsin deficiency: the current state of the evidence. Chronic Obstr Pulm Dis. 2018;6(1):100–xiv.
-
Brantly ML, Wittes JT, Vogelmeier CF, Hubbard RC, Fells GA, Crystal RG. Utilise of a highly purified blastoff 1-antitrypsin standard to institute ranges for the common normal and deficient alpha 1-antitrypsin phenotypes. Breast. 1991;100(iii):703–viii.
-
Tonelli AR, Brantly ML. Augmentation therapy in alpha-1 antitrypsin deficiency: advances and controversies. Ther Adv Respir Dis. 2010;iv(5):289–312.
-
Wewers Doctor, Casolaro MA, Crystal RG. Comparing of alpha-1-antitrypsin levels and antineutrophil elastase chapters of blood and lung in a patient with the alpha-1-antitrypsin phenotype nothing-naught before and during alpha-1-antitrypsin augmentation therapy. Am Rev Respir Dis. 1987;135(iii):539–43.
-
Campos MA, Geraghty P, Holt One thousand, Mendes E, Newby PR, Ma S, et al. The biological effects of double-dose alpha-1 antitrypsin augmentation therapy. A airplane pilot clinical trial. Am J Respir Crit Care Med. 2019;200(3):318–26.
-
Bornhorst JA, Greene DN, Ashwood ER, Grenache DG. alpha1-antitrypsin phenotypes and associated serum protein concentrations in a large clinical population. Chest. 2013;143(4):1000–8.
-
Ferrarotti I, Thun GA, Zorzetto M, Ottaviani S, Imboden M, Schindler C, et al. Serum levels and genotype distribution of alpha1-antitrypsin in the full general population. Thorax. 2012;67(8):669–74.
-
Seersholm North, Kok-Jensen A, Dirksen A. Survival of patients with severe alpha 1-antitrypsin deficiency with special reference to non-alphabetize cases. Thorax. 1994;49(7):695–8.
-
Cagnone M, Piloni D, Ferrarotti I, Di Venere 1000, Viglio S, Magni S, et al. A airplane pilot study to investigate the remainder between proteases and α1-antitrypsin in bronchoalveolar lavage fluid of lung transplant recipients. High-Throughput. 2019;8(ane):5.
-
Janciauskiene S, Welte T. Well-known and less well-known functions of alpha-ane antitrypsin. Its role in chronic obstructive pulmonary illness and other disease developments. Ann Am Thorac Soc. 2016;13(Suppl 4):S280–viii.
-
Janciauskiene SM, Bals R, Koczulla R, Vogelmeier C, Kohnlein T, Welte T. The discovery of alpha1-antitrypsin and its role in wellness and disease. Respir Med. 2011;105(8):1129–39.
-
Paczek 50, Michalska W, Bartlomiejczyk I. Trypsin, elastase, plasmin and MMP-ix activity in the serum during the homo ageing process. Age Ageing. 2008;37(three):318–23.
-
Ehlers MR. Immune-modulating effects of blastoff-i antitrypsin. Biol Chem. 2014;395(x):1187–93.
-
Kawabata K, Hagio T, Matsuoka Due south. The office of neutrophil elastase in astute lung injury. Eur J Pharmacol. 2002;451(ane):one–10.
-
Sanders CL, Ponte A, Kueppers F. The furnishings of inflammation on alpha 1 antitrypsin levels in a national screening cohort. COPD. 2018;15(one):10–half-dozen.
-
Janciauskiene Due south, DeLuca DS, Barrecheguren M, Welte T, Miravitlles Yard, Scientific C, et al. Serum levels of alpha1-antitrypsin and their relationship with COPD in the general spanish population. Arch Bronconeumol. 2020;56(2):76–83.
-
Carroll TP, O'Brien ME, Fee LT, Molloy K, Murrary B, Ramsawak Southward, et al. Alpha-1 antitrypsin deficiency—a missed opportunity in COPD?. IntechOpen; 2014. https://world wide web.intechopen.com/books/copd-clinical-perspectives/blastoff-1-antitrypsin-deficiency-a-missed-opportunity-in-copd-. Accessed 10 Mar 2021.
-
Janciauskiene S, Wrenger South, Immenschuh S, Olejnicka B, Greulich T, Welte T, et al. The multifaceted furnishings of alpha1-antitrypsin on neutrophil functions. Forepart Pharmacol. 2018;9:341.
-
Stockley RA. The multiple facets of blastoff-ane-antitrypsin. Ann Transl Med. 2015;3(10):130.
-
Stockley RA, Bayley DL, Unsal I, Dowson LJ. The effect of augmentation therapy on bronchial inflammation in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2002;165(11):1494–8.
-
Malerba K, Ricciardolo F, Radaeli A, Torregiani C, Ceriani Fifty, Mori Eastward, et al. Neutrophilic inflammation and IL-viii levels in induced sputum of alpha-1-antitrypsin PiMZ subjects. Thorax. 2006;61(2):129–33.
-
Lomas DA. The selective advantage of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2006;173(10):1072–7.
-
Matamala N, Gomez-Mariano K, Lara B, Martinez MT, Rodriguez E, Lazaro Fifty, et al. Neutrophil elastase gene expression and relation with lung function in alpha-1 antitrypsin deficiency patients. Eur Respir J. 2017;l:PA970.
-
Clinical Trials.gov. Alvelestat (MPH966) for the treatment of ALpha-i ANTitrypsin Deficiency (ATALANTa) 2018. https://clinicaltrials.gov/ct2/show/NCT03679598. Accessed ten Mar 2021.
-
Janus ED. Alpha ane-antitrypsin Pi types in COPD patients. Chest. 1988;94(2):446–vii.
-
Lieberman J, Winter B, Sastre A. Blastoff 1-antitrypsin Pi-types in 965 COPD patients. Chest. 1986;89(three):370–3.
-
Topic A, Stankovic M, Divac-Rankov A, Petrovic-Stanojevic N, Mitic-Milikic M, Nagorni-Obradovic L, et al. Blastoff-1-antitrypsin deficiency in Serbian adults with lung diseases. Genet Exam Mol Biomarkers. 2012;xvi(eleven):1282–6.
-
Ortega VE, Li 10, O'Neal WK, Lackey L, Ampleford Eastward, Hawkins GA, et al. The effects of rare SERPINA1 variants on lung office and emphysema in SPIROMICS. Am J Respir Crit Intendance Med. 2020;201(5):540–54.
-
Molloy K, Hersh CP, Morris VB, Carroll TP, O'Connor CA, Lasky-Su JA, et al. Description of the adventure of chronic obstructive pulmonary affliction in alpha1-antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med. 2014;189(4):419–27.
-
Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung affliction: the GOLD scientific discipline committee report 2019. Eur Respir J. 2019;53(5):1900164.
-
Miravitlles Thousand, Dirksen A, Ferrarotti I, Koblizek V, Lange P, Mahadeva R, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in alpha1-antitrypsin deficiency. Eur Respir J. 2017;50(5):1700610.
-
Sandhaus RA, Turino G, Brantly ML, Campos M, Cross CE, Goodman Grand, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis. 2016;3(3):668–82.
-
Casas F, Blanco I, Martinez MT, Bustamante A, Miravitlles M, Cadenas South, et al. Indications for agile case searches and intravenous alpha-1 antitrypsin treatment for patients with alpha-i antitrypsin deficiency chronic pulmonary obstructive disease: an update. Arch Bronconeumol. 2015;51(4):185–92.
-
Attaway A, Majumdar U, Sandhaus RA, Nowacki Equally, Stoller JK. An analysis of the caste of concordance amidst international guidelines regarding alpha-1 antitrypsin deficiency. Int J Chronic Obstr Pulm Dis. 2019;14:2089–101.
-
Stolk J, Tov North, Chapman KR, Fernandez P, MacNee W, Hopkinson NS, et al. Efficacy and safety of inhaled alpha1-antitrypsin in patients with astringent alpha1-antitrypsin deficiency and frequent exacerbations of COPD. Eur Respir J. 2019;54(5):1900673.
-
Horvath I, Canotilho M, Chlumsky J, Chorostowska-Wynimko J, Corda 50, Derom East, et al. Diagnosis and management of alpha1-antitrypsin deficiency in Europe: an proficient survey. ERJ Open Res. 2019;v(1):00171–2018.
-
Esquinas C, Serreri Due south, Barrecheguren M, Rodriguez Eastward, Nunez A, Casas-Maldonado F, et al. Long-term evolution of lung part in individuals with blastoff-i antitrypsin deficiency from the Spanish registry (REDAAT). Int J Chronic Obstr Pulm Dis. 2018;13:1001–7.
-
Wencker K, Fuhrmann B, Banik Northward, Konietzko Northward. Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen. Longitudinal follow-up of patients with alpha(1)-protease inhibitor deficiency earlier and during therapy with Four blastoff(ane)-protease inhibitor. Breast. 2001;119(iii):737–44.
-
Sorheim IC, Bakke P, Gulsvik A, Pillai SG, Johannessen A, Gaarder PI, et al. alpha(1)-Antitrypsin protease inhibitor MZ heterozygosity is associated with airflow obstacle in two large cohorts. Chest. 2010;138(5):1125–32.
-
Holm KE, Mannino DM, Choate R, Sandhaus RA. Genotype is associated with smoking and other key wellness behaviors amidst individuals with alpha-1 antitrypsin deficiency-associated lung disease. Respir Med. 2018;143:48–55.
-
Hersh CP, Dahl Yard, Ly NP, Berkey CS, Nordestgaard BG, Silverman EK. Chronic obstructive pulmonary disease in alpha1-antitrypsin PI MZ heterozygotes: a meta-assay. Thorax. 2004;59(10):843–9.
-
Oelsner EC, Balte PP, Bhatt SP, Cassano PA, Couper D, Folsom AR, et al. Lung part decline in former smokers and low-intensity current smokers: a secondary data analysis of the NHLBI Pooled Cohorts Written report. Lancet Respir Med. 2020;8(i):34–44.
-
Silva GE, Sherrill DL, Guerra South, Barbee RA. A longitudinal study of alpha1-antitrypsin phenotypes and pass up in FEV1 in a community population. Chest. 2003;123(v):1435–40.
-
Seersholm North, Wilcke JT, Kok-Jensen A, Dirksen A. Gamble of infirmary admission for obstructive pulmonary disease in alpha(1)-antitrypsin heterozygotes of phenotype PiMZ. Am J Respir Crit Care Med. 2000;161(i):81–iv.
-
Mehta AJ, Thun GA, Imboden Yard, Ferrarotti I, Keidel D, Kunzli N, et al. Interactions betwixt SERPINA1 PiMZ genotype, occupational exposure and lung office decline. Occup Environ Med. 2014;71(4):234–xl.
-
Eisner MD, Anthonisen Northward, Coultas D, Kuenzli Due north, Perez-Padilla R, Postma D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary illness. Am J Respir Crit Care Med. 2010;182(v):693–718.
-
Silverman EK. Adventure of lung disease in PI MZ heterozygotes. Current status and future research directions. Ann Am Thorac Soc. 2016;13(Suppl 4):S341–5.
-
Blanco I, Bueno P, Diego I, Perez-Holanda S, Casas-Maldonado F, Esquinas C, et al. Alpha-1 antitrypsin Pi*Z cistron frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chronic Obstr Pulm Dis. 2017;12:561–9.
-
Lewis EC. Expanding the clinical indications for blastoff(1)-antitrypsin therapy. Mol Med. 2012;eighteen:957–70.
-
Halpin DM, Tashkin DP. Defining illness modification in chronic obstructive pulmonary disease. COPD. 2009;6(three):211–25.
-
Buist Equally, Sexton GJ, Nagy JM, Ross BB. The upshot of smoking cessation and modification on lung office. Am Rev Respir Dis. 1976;114(ane):115–22.
-
Beck GJ, Doyle CA, Schachter EN. Smoking and lung function. Am Rev Respir Dis. 1981;123(2):149–55.
-
Martin T, Miravitlles M, TelloFurtado Southward. A PI*MS is not always a PI*MS. An example of when genotyping for alpha-1 antitrypsin deficiency is necessary. Pulmonology. 2020. https://doi.org/10.1016/j.pulmoe.2020.06.013.
-
Foil KE, Blanton MG, Sanders C, Kim J, Al Ashry HS, Kumbhare S, et al. Sequencing alpha-i MZ individuals shows frequent biallelic mutations. Pulm Med. 2018;2018:2836389.
-
Mandorfer M, Bucsics T, Hutya 5, Schmid-Scherzer Thou, Schaefer B, Zoller H, et al. Liver disease in adults with alpha1-antitrypsin deficiency. United Eur Gastroenterol J. 2018;6(five):710–8.
-
Schaefer B, Mandorfer M, Viveiros A, Finkenstedt A, Ferenci P, Schneeberger S, et al. Heterozygosity for the alpha-1-antitrypsin Z allele in cirrhosis is associated with more advanced disease. Liver Transpl. 2018;24(6):744–51.
-
Graziadei IW, Joseph JJ, Wiesner RH, Therneau TM, Batts KP, Porayko MK. Increased risk of chronic liver failure in adults with heterozygous alpha1-antitrypsin deficiency. Hepatology. 1998;28(4):1058–63.
-
Zhou H, Fischer HP. Liver carcinoma in PiZ blastoff-1-antitrypsin deficiency. Am J Surg Pathol. 1998;22(vi):742–8.
Acknowledgements
Medical writing back up was provided by Laura Wesley of Meridian HealthComms Ltd in accordance with adept publication practice (GPP3).
Funding
Medical writing assistance for this manuscript was funded by CSL Behring; the role of the funder was to support medical writing assistance just.
Writer information
Affiliations
Contributions
IB and MM contributed every bit to the concept and development of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approving and consent to participate
Not applicative.
Consent for publication
Not applicable.
Competing interests
IB has received consulting fees from Astra Zeneca, Boehringer Ingelheim, CSL Behring, Grifols, Verona Pharma, GE Healthcare, Mylan, Theravance, GSK and has received research grants from AMGEN, GE Healthcare, Theravance and Mylan. MM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi and Grifols and research grants from GlaxoSmithKline and Grifols.
Boosted data
Publisher'south Notation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open up Access This article is licensed under a Artistic Eatables Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, equally long as you give appropriate credit to the original writer(due south) and the source, provide a link to the Creative Commons licence, and signal if changes were fabricated. The images or other third party material in this article are included in the commodity's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Artistic Eatables licence and your intended use is not permitted past statutory regulation or exceeds the permitted use, y'all volition need to obtain permission direct from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Artistic Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this commodity, unless otherwise stated in a credit line to the information.
Reprints and Permissions
Near this article
Cite this article
Barjaktarevic, I., Miravitlles, Chiliad. Blastoff-ane antitrypsin (AAT) augmentation therapy in individuals with the PI*MZ genotype: a pro/con fence on a working hypothesis. BMC Pulm Med 21, 99 (2021). https://doi.org/ten.1186/s12890-021-01466-x
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s12890-021-01466-10
Keywords
- Alpha-i antitrypsin deficiency
- Genotype
- PI*MZ
- Pulmonary disease
- Chronic obstructive pulmonary disease
Source: https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-021-01466-x